Recommended Global Pharmaceutical Sciences Webinars & Conferences
Europe & UK
Asia Pacific & Middle East
Canada
Asia Pharma 2022
ABOUT CONFERENCE
The pharma is the art and science of preparing, compounding and dispensing medications. It also includes the more modern services related to the health care and the provision of drug and related information to the public. A drug is an ingredient that acts on the living form to alter the bodily process and are used for the prevention, diagnosis and treatment of diseases.
Pharma companies deal in generic or brand medications and medical devices. They are subject to a diversity of laws and regulations that administer the patenting, testing, safety, efficacy & marketing of drugs.
Asia Pharma 2022 Conference is a multidisciplinary program with expansive cooperation with individuals from around the world concentrated on finding out about Pharma Research and its advances. This is your best chance to achieve the biggest gathering of members in our Pharma conference from the scholarly world, inquire about substances, medicinal gatherings, related affiliations, social orders and furthermore from government organizations, pharmaceutical and biomedical research.
Meet your target market with individuals from around the globe concentrated on finding out about Pharma, this is your single best chance to achieve the biggest array of members in our Pharma conference. Direct showings, disperse data, meet with flow and potential Scientists, make a sprinkle with another examination, and get name acknowledgment at this 2-day Pharmaceutical Event. Incredibly famous speakers, the latest methods, strategies, and the most up to date refreshes in Pharmaceutical Sciences fields are signs of this meeting.
SESSIONS AND TRACKS
Track 1: Regulatory Affairs and Intellectual Property Rights
Regulatory Affairs contributes essentially to the overall success of drug development, both at early pre-marketing stages and at all times post-marketing. The pharmaceutical industry deals with an increasing number of interesting drug candidates, all of which necessitate the involvement of the quality assurance in regulatory affairs department.
The importance of intellectual property law is well established at all levels-statutory, administrative and judicial. It lays down minimum standards for protection and enforcement in member countries which are required to promote effective and adequate protection of intellectual property rights with a view to reducing distortions and impediments to international trade.
The Agreement provides norms and standards in respect of following areas of intellectual properties are Patents, Trademarks, copyrights, Geographical indications, Industrial designs.
Track 2: Pharmacognosy and Phytochemistry
Pharmacognosy, a long settled pharmaceutical science, has assumed a various part in the disclosure, characterization, generation and institutionalization of these medications. The importance of this train regarding examination and instructing has expanded in the most recent decade as individuals from the general population in created nations have swung to the utilization of home grown solutions for the self-medicine of minor ailments. In any case, numerous phytomedicines require advance examination for their clinical adequacy, while others should be altogether researched for their potential wellbeing dangers or connections with physician recommended drugs.
Phytochemistry is naturally huge by assuming a basic part in the plants to guard themselves against different pathogenic microorganisms by demonstrating the antimicrobial action by hindrance or executing systems. The discharge of these mixes is differing from plant to plant some deliver increasingly and some create in insignificant amount. Now and then they can be unsafe and some of the time they can be exceptionally useful.
Track 3: Pharmaceutics and Pharmacotherapeutics
Pharmaceutics encompasses a molecular evaluation of drug metabolism and transport processes and the study of genetic, environmental and disease-related factors that regulate or perturb those processes, as well as the fundamental mathematical relationships between enzyme/transporter function, blood concentration-time profiles and the spectrum of pharmacological effects.
Pharmacotherapeutics manages the helpful uses and impacts of medications. Pharmacists are specialists in pharmacotherapy and are in charge of guaranteeing the protected, suitable, and prudent utilization of pharmaceutical medications. As pharmacotherapy masters, pharmacists have duty regarding direct patient care, regularly working as an individual from a multidisciplinary group, and going about as the essential wellspring of medication related data for other social insurance experts. A pharmacotherapy expert is a person who is spent significant time in managing and endorsing medicine, and requires broad scholarly information in pharmacotherapy.
Track 4: Nanomedicine and Nanotechnology
Nanomedicine was conceived as a consolidated consequence of the various inquires about done agony staking by a huge number of researchers over the globe. At the point when the researchers ended up plainly mindful of its innumerous applications in pharmaceutical, its significance came to more up to date statures. Presently the whole restorative group everywhere throughout the world is looking forward excitedly for the supernatural occurrences of nano drugs. The primary fascination of this novel innovation is its adaptability for advancements. Another critical part of this is dissimilar to numerous other advancements which the conventional man can't stand to utilize, nanomedicine can undoubtedly extend its application to each areas of the general public.
Nanotechnology brings together scientists and engineers from many different subjects, such as applied physics, materials science, interface and colloid science, device physics, chemistry, Supramolecular chemistry (which refers to the area of chemistry that focuses on the non-covalent bonding interactions of molecules), self-replicating machines and robotics, chemical engineering, mechanical engineering, biology engineering, and electrical engineering.
Track 5: Industrial and Physical Pharmacy
Industrial pharmacy is a discipline which includes manufacturing, development, marketing and distribution of drug products including quality assurance of these activities. The research topics are focussed on solving current general problems in pharmaceutical industry, such as formulation and characterisation of sticky amorphous drugs, problem-solving for paediatric medicines and miniaturisation of manufacturing processes.
Physical pharmacy incorporates information of arithmetic, material science and science and applies them to the pharmaceutical dose frame improvement. Physical pharmacy gives the premise to understanding the synthetic and physical wonders that oversee the in vivo and in vitro activities of pharmaceutical items.
Track 6: Pharmacology and Toxicology
Pharmacology is the study of how medicine and other things have an effect on living organisms and change how they function. The field encompasses drug composition and properties, synthesis and drug design, molecular and cellular mechanisms, organ/systems mechanisms, signal transduction/cellular communication, molecular diagnostics, interactions, chemical biology, therapy & medical applications and anti-pathogenic capabilities.
Toxicology manages the new thoughts to shield people and the earth from the hurtful impacts of toxicants and furthermore to empower the development of additionally perceiving toxicants, for example, anticancer drugs, clinical medications and pesticides. Toxicology is exceedingly assorted science and human movement attracting from and adding to a wide scope of different sciences and human occasions. Furthermore, one of those is the sciences that contribute their strategies and philosophical ideas to serve the requirements of toxicologists, either in research or in the use of toxicology to human issues.
Track 7: Pharmaceutical Chemistry
Pharmaceutical Chemistry is a core discipline within Pharmaceutical Sciences. It is centrally engaged in the drug discovery process, mainly focusing on lead finding, lead optimisation and structure-activity relationship investigations, using technologies of computer-aided drug design, natural products chemistry, synthetic organic chemistry, and biochemical molecules approaches in a trans-disciplinary combination which is generally known by the term "Medicinal Chemistry".
The pharmaceutical market was esteemed at $1 trillion in 2013, as indicated by IMS. The market developed at a 4% compound yearly development rate (CAGR) from 2009 to 2013. It developed well in 2010 and 2011 preceding a decrease in development in 2012 and 2013.
Track 8: Pharmaceutical Analysis
Pharmaceutical analysis is a process or a sequence of processes to identify and/or quantify a substance or drug, the components of a pharmaceutical solution or mixture or the determination of the structures of chemical compounds used in the formulation of pharmaceutical product. Analytical techniques mainly used for the separation of the components from the mixture and for the determination of the structure of the compounds.
The worldwide income of the statistical surveying industry surpassed 40 billion U.S. dollars in 2013, rising year-on-year since it encountered a slight plunge in 2009 amid the Great Recession. In 2013, Europe produced the biggest offer of statistical surveying income at 40 percent, or 16 billion U.S. dollars, nearly taken after by North America with 39 percent. Notwithstanding contributing the most income that year, Europe additionally observed the biggest decrease in income over the earlier year, falling by 1.4 percent.
Track 9: Pharmaceutical Microbiology
It includes the research and development of anti-infective agents, the use of microorganisms to detect mutagenic and carcinogenic activity in prospective drugs, and the use of microorganisms in the manufacture of pharmaceutical products like insulin and human growth hormone.
Pharmaceutical microbiology is additionally involved with the validation of disinfectants, according to standards, to evaluate the efficacy of disinfectants in suspension, on surfaces, and through field trials.
The consumables, hardware and innovation advertises in the microbiology business totalled about $7.7 billion in 2012. This aggregate is relied upon to develop from $8.5 billion in 2013 to $11.4 billion in 2018, with a compound yearly development rate (CAGR) of 6.1% for the five-year time frame, 2013 to 2018.
Track 10: Pharmaceutical Biotechnology
Pharmaceutical biotechnology has emerged as one of the major disciplines for drug discovery and development. Today the shape and vision of pharmaceutical aspects and challenges have completely changed, and the prefix "pharma" can also be accepted as a synonym for integrated life science approaches, ranging from genetics to molecular biology to diagnostics, with the common goal of delivering the best drug to the patient by biotechnological techniques.
Worldwide market for biotechnology in the life sciences is evaluated at over $29.6 billion for 2014. Pharmaceutical Biotechnology will demonstrate unfaltering managed development at 20.7%. This market is conjecture to develop to more than $79.8 billion by 2019 to enlist a solid compound yearly development rate (CAGR) of 22%.
Track 11: Biochemistry and Molecular Biology
By controlling information flow through biochemical signalling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last 40 years, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine are engaged in biochemical research.
Today, the main focus of pure biochemistry is in understanding how biochemical molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms. Biochemistry has advanced, especially since the mid-20th century, with the development of new techniques such as chromatography, X-ray diffraction, dual polarisation interferometry, NMR spectroscopy, radio isotopic labelling, electron microscopy, and molecular dynamics simulations. These techniques allowed for the discovery and detailed analysis of many molecules and metabolic pathways of the cell, such as glycolysis and the Krebs cycle.
The report examinations the overall markets for Laboratory Chemical Reagents in US$ Million by the fragments like Biochemistry and Molecular Biology. Yearly gauges and figures are accommodated the period 2013 through 2020. Work of organic chemists and biophysicists is anticipated to grow 8 percent from 2014 to 2024, about as quick as the normal for all occupations.
Track 12: Clinical and Hospital Pharmacy
The study of pharmacy involves the effective recommendation and administration of various medications for the safety and health of patients. Hospital pharmacy is a specialization of this field that includes additional duties such as aiding doctors in applying drug therapy. The statements were developed by the profession to bring uniformity to the practice of hospital pharmacy.
Recognition of the practice of clinical pharmacy by the profession has led to our professional bodies publishing statements aimed at standardizing pharmacy practice. These statements were developed by the profession to bring uniformity to the practice of clinical pharmacy. However they outlined what pharmacists want from clinical pharmacy, there was little input from clinical colleagues or patients about what they wanted from clinical pharmacy.
Track 13: Drug Safety and Pharmacovigilance
Drug safety is otherwise called Medication Safety in the field of health. It is related with antagonistic impacts of Pharmaceutical items including numerous other logical perspectives, for example, the reactions of medications, the nature of solutions, prescription mistake in utilization of medications, absence of viability of medications, and fake medications. Tolerant Safety, Drug Interaction (drug–drug and food–drug cooperation) Drug Pharmacokinetic, and Adverse Drug Reaction are a few terms required with Drug Safety.
Pharmacovigilance is the science and exercises identifying with the discovery, evaluation, comprehension and counteractive action of unfavorable impacts or some other pharmaceutical related issue. Pharmacovigilance underpins general wellbeing programs by giving solid data to the productive evaluation of the hazard advantage profile of solutions, add to the appraisal of formal, uses, symptoms, damage, viability and danger of pharmaceuticals, empowering the sheltered, sound and more viable utilization of different medications. Advance instruction, understanding and clinical preparing in Pharmacovigilance and its successful accessibility to people in general.
Track 14: Clinical Trials and Research
Clinical trials are research concentrates that investigate whether a restorative technique, treatment, or gadget is protected and compelling for people. Contemplate indicate which therapeutic methodologies work best for specific ailments or gatherings of individuals. The reason for clinical trials is research, so the reviews take after strict logical models. These measures ensure patients and help deliver dependable review comes about. Clinical trials are one of the last phases of a long and watchful research prepare.
Clinical research concentrates to explore whether a remedial system, treatment, or device is ensured and effective for individuals. Consider show which therapeutic strategies work best for particular afflictions or gatherings of people.
Track 15: Pharmacogenetics and Genomics
Pharmacogenetics is the science that supports understanding the part that a person's hereditary make-up plays in how well a prescription functions, and additionally what symptoms are probably going to happen, enhancing our capacity to distinguish the hereditary reasons for illnesses and look for new medication targets. Pharmacogenetics alludes to hereditary contrasts in metabolic pathways which can influence singular reactions to drugs, both as far as restorative impact and additionally antagonistic impacts.
Pharmacogenomics is a quickly creating field that has essential ramifications in individualized treatment for patients and its suggestion influence tranquilize advancement issues such as medication safety, efficiency, and customized health care. Pharmacogenomics consolidates customary pharmaceutical sciences, for example, natural chemistry with explained colleague of qualities, proteins, and single nucleotide polymorphisms.
Track 16: Green and Community Pharmacy
Green Pharmacy is proposed for the need of synthesis from raw materials, production of products, transportation, storage, deliveries, usage, and disposal, are appropriately assessed, anticipated, and managed. Clearly, pharmacists and the practice of the broader pharmaceutical sciences must contribute to achieving a greener pharmacy.
The practice of community pharmacy has extended critical part in giving protected, viable medicine treatment and directing on proper utilize and precautionary measures. Today's people group drug specialists are expecting a more extensive part in advancing health—avoiding malady, enhancing nature of care, and expanding access to mind. Community pharmacists are extending their administrations inside the drug store and past all through the moves of care, working inter professionally to address the issues of the changing medicinal services scene.
Track 17: Nutraceuticals and Natural Medicine
Nutraceuticals are bioactive constituents that maintain or advance wellbeing and happen at the convergence of nourishment and pharmaceutical ventures. Such substances may run from segregated supplements, dietary supplements and particular weight control plans to hereditarily built home grown items, prepared nourishments and drinks. They have gigantic effect on the medicinal services framework and may give therapeutic medical advantages including the counteractive action or potentially treatment of maladies and physiological issue. These phytochemicals, either alone or additionally in mix, have enormous remedial potential in curing different afflictions.
Natural medicine offers safe, cost-effective solutions for many of our nation's healthcare problems. Competence and respect for tradition, the scientific method, and innovation are hallmarks of a naturopathic medical practice.
Track 18: Pharmacy Education and Practice
Standards of initial education and training for pharmacists set out the criteria against which we will approve education and training for student pharmacists and pre-registration trainee pharmacists. The standards ensure that newly registered pharmacists are competent to practice safely and effectively. The mission of pharmacy education is to prepare graduates who provide patient centered care that ensures optimal medication therapy outcomes and provides a foundation for specialization in specific areas of pharmacy practice; to participate in the education of patients, other health care providers and future pharmacists, to conduct research and scholarly activity and to provide service and leadership to the community.
Recommended Pharma Conferences | Pharma Meetings | Pharma Events
Related Pharma Societies:
American Association of Pharmaceutical Scientists (AAPS), British Columbia Pharmacy Association, Canadian Pharmacists Association (CPhA), Drug Information Association, Kuwait Pharmaceutical Association.
Track 19: Pharmaceutical Care and Health Systems
Pharmaceutical Care is a patient-centered, outcomes oriented pharmacy practice that requires the pharmacist to work in concert with the patient and the patient's other healthcare providers to promote health, to prevent disease, and to assess, monitor, initiate, and modify medication use to assure that drug therapy regimens are safe and effective.
A good health system delivers quality services to all people, when and where they need them. The exact configuration of services varies from country to country, but in all cases requires a robust financing mechanism; a well-trained and adequately paid workforce; reliable information on which to base decisions and policies; well maintained facilities and logistics to deliver quality medicines and technologies.
Track 20: Pharma Manufacturing and Compliance
Pharma manufacturing involves industrial-scale synthesis of pharmaceutical drugs by pharmaceutical companies including series of unit operations, such as milling, granulation, coating, tablet pressing and others.
The pharmaceutical industry has provided the world with incredible medical breakthroughs. Although science continues to outpace the law, it is imperative that compliance infrastructures remain current. Effective compliance programs continue to be top of mind for industry stakeholders. It is important that regulatory compliance programs continue to ensure that training programs are designed to effectively train management including the directors of life sciences companies.
MARKET ANALYSIS
The worldwide market for pharmaceuticals came to $1.2 trillion out of 2018, up $100 billion from 2017, as indicated by the Global Use of Medicines report from the IQVIA Institute for Human Data Science. Going ahead, the worldwide market will develop by 4-5% CAGR, coming to $1.5 trillion (in light of receipt evaluating). That rate is lower than the 6.3% CAGR of the 2014-2018 period.
For the US explicitly, the 2018 spending was $485 billion, up 5.2% over the earlier year, and the 2023 spending will be $625-655 billion, speaking to a 4-7% CAGR over the five-year time frame. For quite a while now, IQVIA has been mindful so as to recognize "receipt" income (pretty much proportional to list estimating) from "net" income—the income that really gathers to the pharma business after discounts and conclusions are calculated in. US net income is relied upon to have risen 1.3% during 2018, and at a 0-3% rate throughout the following five years.
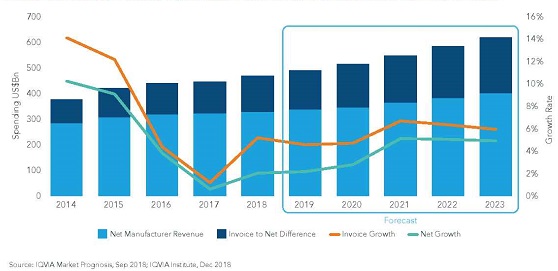
Asian pharmaceutical advertise is ascending at a very quicker rate. As far as development, Singapore is turning into a worldwide place for pharmaceutical industry. Pharmaceutical assembling and generation industry assumes a significant job in Singapore's economy. In 2012, according to World Bank information, Singapore burns through 4.7% of its GDP into medicinal services industry. Also, pharmaceutical industry contributes over 85% of absolute biomedical sciences fabricating yield of Singapore yearly. According to World Health Organization (WHO), Singapore's medicinal services framework holds 6th position around the world.
Singapore's pharmaceutical segment pulls in numerous outside organizations for ventures. For example, in excess of 30 prescription tech organizations have put for explore and advancement and in setting up pharmaceutical assembling plants in Singapore. Singapore's worldwide companies and neighborhood new businesses are biggest producers and exporters of medicinal hardware over the world. A few therapeutic items are traded from Singapore, for example, syringes, catheters, look into instruments and logical expository gear. Over 70% of world's smaller scale clusters, 10% of worldwide contact focal points and half of worldwide warm cycles and mass spectrometers are produced in Singapore.
TOP 10 REASONS TO ATTEND OUR ASIA PHARMA 2022
A unique once a year opportunity:
Around the globe, there is no other conference which provides the depth of the information in Pharmaceutical Sciences.
Learn New Trends Strategies in research.
New technology and tools are exclusively discussed at our conference. Don’t let old & outdated information hold you back.
Frank Talks:
The International speakers can discuss on what is the future of Pharmaceutical Industry.
Network and Connect:
Meet and exchange ideas with hundreds of like-minded professionals who are leaders in Pharma Industry.
Improve your Results:
Learn new skills to learn to deepen your relationships with clients and donors that will bring greater results for your research work and business.
Get Inspired:
Our speakers and attendees are highly skilled and experienced professionals inspirational in their passion to make a long-term positive impact.
Sample and Compare the Tools:
Connect with vendors and exhibitors across the globe to learn about products and services. And check out the latest tools and ideas.
Value for the Money:
With two-day intensive conference schedule, you will access strategic gift planning knowledge and expertise that’s worth its weight golf form an impressive array of recognized professionals.
Spread the Impact:
Share the healing knowledge and new ideas with our organization.
Join a special collaboration:
For 10 years, the planned giving community from across the globe has been meeting once a year to learn network and brainstorm ideas on how to treat the diseases with healing. Join us to collaborate.
To Collaborate Scientific Professionals around the World
Conference Date November 21-22, 2022
For Sponsors & Exhibitors
Speaker Opportunity
Useful Links
Past Conference Report
Supported By
All accepted abstracts will be published in respective Conference Series International Journals.
Abstracts will be provided with Digital Object Identifier by











